Advertisement
Surgeons Challenge FDA Warnings On Vaginal Mesh
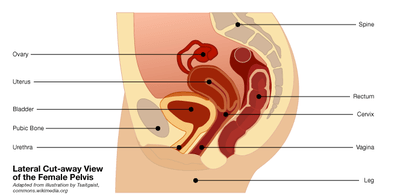
The debate over a controversial medical device used for women is heating up.
A nationwide coalition of pelvic surgeons is challenging a recent U.S. Food and Drug Administration safety advisory that warns of serious risks, complications and questionable benefits of surgical mesh implanted vaginally to treat women for a condition known as prolapse. The procedure is used to strengthen vaginal tissue that has grown weak or stretched, often after childbirth.
In advance of an FDA advisory panel meeting Sept 8-9 to discuss the safety and effectiveness of the mesh — and as attorneys across the country intensify their efforts targeting the device through litigation — pelvic surgeons say they e-mailed a response letter to the FDA outlining their concerns. A copy of the letter was obtained by CommonHealth.
"We have some significant disagreement with the conclusions reached [by The FDA] and concerns regarding the message that it is sending to our patients, the healthcare community, and unfortunately to the legal community as well," says the Aug. 25 letter, which is signed by more than 600 pelvic surgeons, according to one of the organizers, Dr. Miles Murphy, associate medical director at the Institute for Female Pelvic Medicine & Reconstructive Surgery and Director of the Division of urogynecology at Abington Memorial Hospital in Pennsylvania.
Dr. Murphy, also an assistant clinical professor at Temple University School of Medicine, says he hopes the letter will "offer balance" to the FDA panel discussions next week. "It seems like the information that the FDA is working with now is very skewed," Murphy said in an interview. "I'm hoping that the information [in our letter] will allow us to continue doing a procedure that in the right hands can be very beneficial."
While acknowledging the risks involved in this and, indeed, all types of operations, the surgeons write that portions of the FDA's analysis are flawed and the agency is unfairly portraying this particular type of mesh surgery as uniquely hazardous, when, in fact, for certain patients, it is an effective tool and "may be the best option," the letter says.
Karen Riley, a spokesperson for the FDA, said she hadn't yet seen the letter. But, in preparation for the meeting next week, she said the agency will post an "executive summary" of the issues related to vaginal mesh on its website tomorrow, which will include more details about safety findings, and other new information.
Here's a little history:
In July, the FDA updated a 2008 safety alert after a rise in reported complications from vaginally implanted surgical mesh to treat pelvic organ prolapse, which occurs when the tissues that hold the pelvic organs in place become weakened or stretched out. In prolapse, internal organs such as the uterus, bladder or rectum can slip out of place and bulge into the vagina, sometimes past its opening. It's bad news, but it can be treated in many ways, depending on the patient, using both surgical and non-surgical methods.
(For context: Prolapse can also be treated using surgical mesh through the abdomen, which the FDA says appears to have fewer complications. Additionally, the procedure can be done without mesh, either abdominally or vaginally, and is known as native tissue repair. Both FDA safety advisories — the July update and the initial 2008 alert — focused on vaginally implanted mesh for prolapse, but the 2008 alert also included mesh for stress urinary incontinence. That's another story.)
The FDA said the most frequent complications reported for surgical mesh devices used in prolapse repair include "mesh erosion through the vagina (also called exposure, extrusion or protrusion), pain, infection, bleeding, pain during sexual intercourse, organ perforation, and urinary problems. There were also reports of recurrent prolapse, neuro-muscular problems, vaginal scarring/shrinkage, and emotional problems." The agency said that many of the complications required additional medical or surgical treatment and hospitalization.
In their letter, which Dr. Murphy says has also been submitted in a shortened manuscript version to the International Urogynecology Journal, the surgeons disagree with several of the FDA's conclusions. Here are some of the major issues:
1. The Complication Rate
The FDA's July update says that: "Serious complications associated with surgical mesh for transvaginal repair of POP [prolapse] are not rare." It says in 2010 at least "100,000 POP repairs that used surgical mesh were performed" and "75,000 of these were transvaginal procedures." The FDA cites 1503 reports of complications associated with the transvaginal procedure from 2008-2010.
The surgeons write that they believe this number "doesn't represent an increase in the rate of complications; rather it is a reflection of the wide acceptance [of the procedure] by many specialists in POP surgery and an increase in the overall rate of how often these procedures are being performed." In other words, more widespread surgery equals more reports of complications.
2. Greater Risk
The FDA says transvaginal mesh surgery "may expose patients to greater risk" than traditional non-mesh repairs. The surgeons say this assertion is "unproven by the existing literature," and note that complications associated with medical devices (like mesh) are reported to the FDA --while complications from surgery not involving devices are not — so the agency is simply unable to compare apples to apples here.
The surgeons say they don't want to minimize the risk of surgical complications, but:
"We...wish to highlight a fundamental reality within our daily surgical practices that we believe is not reflected or acknowledged in the current FDA verbiage; that is, in the surgical management of advanced prolapse, all treatment options involve significant risks. We are deeply concerned that the UPDATE portrays transvaginal mesh repairs as uniquely hazardous, providing no broader perspective (quantitatively or qualitatively) regarding the significant risks and/or higher recurrence rates associated with its alternatives. This introduces a huge clinical dilemma, and unjustified medical-legal exposure, for well-trained surgeons serving the vast 'denominator' of women undergoing mesh procedures."
3. Erosion and Contraction
The FDA says that "both mesh erosion and mesh contraction [a previously unidentified risk where the mesh shrinks in the body] may lead to severe pelvic pain, painful sexual intercourse or an inability to engage in sexual intercourse. Also, men may experience irritation and pain to the penis during sexual intercourse when the mesh is exposed in mesh erosion."
In response, the surgeons say while erosion is a potential complication, it can almost always be resolved. "We are unaware of any published case reports in which mesh erosion from TVM does not resolve after more than two returns to the operating room," the letter says.
Dr. Peter Rosenblatt, Director of Urogynecology and Reconstructive Pelvic Surgery at Mt. Auburn Hospital, a teaching hospital of Harvard Medical School in Cambridge, Mass., and one of the letter's co-signers, told me in an interview that he doesn't consider erosion a serious complication and feels it's wrong that the FDA lumps it into that category. "It's more of a nuisance than anything else," he says.
4. A Flawed View
The surgeons write that "a fundamental flaw in the FDA's analysis is that it is based on the question of proof of superiority of mesh in all patients." But, they write: "No one is suggesting that mesh is recommended in all patients."
Samantha Pulliam, M.D. associate director of the Division of Urogynecology at Massachusetts General Hospital in Boston told me she uses transvaginal mesh for prolapse in very few patients. "I think it's a very rare circumstance where it's truly indicated," she said. "I like to choose the thing that will last the longest, work the best and be safest, that's my feeling."
"From our vantage point," the surgeons' letter continues, "it appears the FDA has presented a biased view of [trans-vaginal mesh] among all [prolapse] repair procedures because of the current reporting mechanisms in place...in our opinion, some of the statements made in the UPDATE would also contribute to further suffering by depriving our patients of surgical options that may be in their best interest."
The FDA makes clear that the complications associated with mesh, which is produced by a number of different manufacturers, have not been linked to any single brand.
Still, the mesh manufacturers are centrally involved here. (Dr. Murphy told me no mesh makers had any part in the letter-writing process.)
I called Boston Scientific, of Natick, Mass., one of the mesh makers, and they referred me to the trade group for medical devices, the Advanced Medical Technology Association, AdvaMed. Here's their statement:
"For women suffering from pelvic organ prolapse or stress urinary incontinence, surgical intervention and the use of mesh is an important treatment option. The use of surgical mesh has an established track record... Collaboration among medical device companies, physicians and regulatory bodies over the years has supported and sustained patient safety and innovation. We look forward to continuing to discuss this matter with FDA and other stakeholders at the upcoming advisory panel."
Clearly, this is not the end of the mesh story.
Personal injury lawyer Mark Mueller, of Austin, Texas says he's got about 1,000 mesh cases moving along at various stages in the legal process. He says he believes women are not fully aware of the risks of surgical mesh. "This, he says, "is just the tip of the iceberg."
Readers, do you have mesh stories to report? I know this post includes no patient voices, and I know we need them. So please contact me, or post your thoughts, as we will be covering this issue as it unfolds.
This program aired on August 31, 2011. The audio for this program is not available.
