Advertisement
MIT Lab Hosts Nation's First Stool Bank, But Will It Survive?
You walk through a labyrinth of MIT buildings and into what looks like a typical laboratory: white walls and clean counters, the constant buzz of machines, the clutter of pipettes. In the corner, you open the door to a hulking freezer.
When the puff of frosty air clears, you see stacks of plastic bottles filled with what looks a little like smoothies — in tawny, rusty colors Odwalla would never market. That's your first hint of this lab's unique purpose. Then there's the giveaway: on the sterile countertop, you see a trophy of a squatting muscleman, labeled "Most Generous Donation."
Welcome to the first national stool bank. It’s like a blood bank, but for fecal matter. And that brown smoothie is actually very healthy stool, parasite-free and loaded with happy bacteria.
In early October, the stool bank — called OpenBiome — started shipping these bottles around the country. Once that FedEx box of dry ice and stool arrives at the hospital, the doctor can do a fecal transplant — which is exactly what it sounds like. You take a healthy person’s feces and put them into a very sick person’s gut. And if all goes well, a few hours later that sick person is much better.
America is just beginning to develop a stomach for this procedure — it's gaining popularity among patients and doctors. And by all accounts the stool bank has made things much easier. But there is a chance those stool shipments will come to an abrupt halt.
Late last week, the FDA released a draft of its new fecal transplant guidelines. As they are worded, things don’t look good for the OpenBiome stool bank.
The FDA is thinking about requiring the patient or the doctor to personally know the donor. But that doesn’t work so well for the stool bank, where the donations come from "Donor One" and "Donor Two." They are anonymous gifts and soon that might not be allowed.
The Ecosystem In Our Gut
But, first things first, why are we even talking about poop transplants?
It all goes back to the fact that there are 100 trillion bacteria living inside us. That means there are 10 times more of them than there are human cells in our body. Together, they are called the microbiome.
Research on the microbiome is still in its early phases. But we do know one thing for sure — we can’t live happily without a healthy mix of bacteria.
"There is a little rainforest inside of us and it’s keeping us healthy and helping us digest food," said Mark Smith, a PhD student at MIT and one of the founders of OpenBiome. "And normally if you were to unleash some weed(s) in the rainforest — it’s super dark and there’s no room for a new organism to start to grow. They will be competed out by all the things that are already there.”
But if “you go in and you clear-cut it with antibiotics," he said, and then let some invasive species of weed loose, "it’s going to grow really, really quickly.”
Death from Diarrhea
That’s basically what happens with a bacterium called C. diff — short for Clostridium difficile.
Usually, C. diff is just one weed in our gut’s rainforest. But when strong antibiotics burn down most of our forest, C. diff can easily take over. And C. diff isn’t one of those benevolent bacteria. It releases toxins that give people dreadful diarrhea.
Recently C. diff has been gaining gut real estate. It’s become the most common hospital-acquired infection in the country. Roughly half a million people get it every year and 14,000 of them die.
Catherine Duff, a mother of three from Indiana, is one of those half million. Back in 2005, she got C. diff. Since then, she has had it a gut-wrenching eight times. Each time it gets worse.

Duff says she was “ill to the point of basically not getting out of bed, unless it was to go to the bathroom again. I was going to the bathroom 20 to 30 times a day. Going to the hospital about every other day for IV fluids.”
And she was losing hope.
“I was out of options," she said. "I was dying. I had basically gotten to the point that my quality of life was so bad that I was actually beginning to think that death would be better.”
Duff was so sick she had a team of eight doctors. They tried all the drugs they could think of and, eventually, decided the only option was to remove her colon.
In desperation, one of Duff’s daughters went online and happened to stumble across fecal transplants. None of Duff’s eight doctors had mentioned it. And only two of them had ever heard of it.
Fecal Transplants
Catherine Duff printed out all the information she could find and brought it to her doctors. All refused to do it.
But after enough pestering, one doctor agreed to test her husband for parasites, HIV, and other things that might be transmitted by sharing poop.
This donor testing can be expensive, said Michael Edmond, a professor of infectious diseases at Virginia Commonwealth Medical Center in Richmond and one of a small number of doctors willing to do fecal transplants.
The donor screening isn’t covered by insurance “because the donor does not have any disease," he said. "So, the out-of-pocket cost for that is in the range of $750 to $1,500.”
The cost varies because there is no official list of which tests should be done. Each doctor and patient creates their own screening standards.
After Duff’s husband passed their self-designed screening, the couple went home to do the transplant in their bathroom. After her husband produced a sample, they did some mixing, blending and straining, then used an enema to introduce it into Catherine Duff's bowels.
“We did it at 4 p.m. one afternoon," Catherine Duff said. "By about 10 o’clock that night, I felt good. I slept through the night for the first time. And the next morning, I had enough energy to get up and take a shower and get dressed and go downstairs and eat. And these were all things I had not been able to do in months.”
Dr. Edmond says this is pretty common. The vast majority of his patients are better within a day — remarkable, he says, given that many of them have suffered from debilitating diarrhea for months, if not years or decades. Research shows that for C. diff, fecal transplants work in about 90 percent of cases.
"If you look at the literature — and there’s about 600 or more cases that have been formally reported in the literature — you’d be hard-pressed to find any adverse event that’s occurred because of fecal transplantation,” Dr. Edmond said.
And the benefits might not be limited to patients with C. diff. Fecal transplants may also help those with Crohn’s Disease or other inflammatory bowel diseases.
Reluctant Doctors
So, it’s effective. It’s cheap. It’s easy. But that doesn’t mean it’s popular among doctors.
'There’s a lot of concern about spraying poop all over a hospital. Nobody wants that to happen.'
Logistically, it’s a challenge. Not only does the patient have to find a donor and the doctor have to screen them, the donor has to perform on command. If they can’t move their bowels at the right moment, things have to be rescheduled.
But if they do produce a generous donation, then the doctor needs space and a technician to prepare it.
As Smith, from OpenBiome, explains this can make doctors nervous: "A hospital needs to be a very clean environment. And you are talking about taking poop, putting it in a blender and pressing go...There’s a lot of concern about spraying poop all over a hospital. Nobody wants that to happen.”
So, you need to have a special space designated for fecal transplants. Otherwise, there are all sorts of added liability concerns about feces ending up in the wrong place.
And then, Dr. Edmond says, there is the money issue: not only does the patient have to pay out of pocket for stool screening, but the doctor cannot get reimbursed for the procedure.
“There wasn’t even a [billing] code for it until January of this year. But the code isn’t associated with any reimbursement. So we are not being paid to do it. If you're talking about something that takes up three hours of your time and you’re not getting paid for that, most doctors really aren’t going to be willing to do that. And in that three hours some of my colleagues have cranked out nine or 10 patients that they were paid for.”
Despite these hurdles, the number of doctors willing to try fecal transplants has begun to grow. Duff collects a list of fecal transplant providers. When she needed her own transplant she could only find one doctor in the country — in Nevada. Now, she has a list of almost 100. And she says almost every day she gets a call from a doctor who wants to start doing fecal transplants.
But these doctors aren’t evenly distributed. If you are on one of the coasts, you are good to go. But in the heartland, it’s much harder to find a doctor willing to do a fecal transplant. Dr. Edmond has patients who have traveled eight hours to get the procedure. And that’s a lot because traveling can be hard while having diarrhea.
Hesitant doctors have meant the Do-It-Yourself movement has flourished. Plenty of YouTube videos and blogs explain the nitty gritty. And, unlike a drug, people have fairly easy access to stool. So it’s hard to mandate oversight.
Smith says that “a lot of patients are being driven underground and doing this in their basement.”
But this has some people worried. What if the recipient doesn’t actually need a fecal transplant? What if the donor isn’t screened properly? What if something goes wrong during the procedure?
No. 1 In No. 2
Two years ago, Mark Smith had a friend in need. After a gallbladder operation, the friend contracted a miserable case of C. diff. Eighteen months and countless drugs later, he decided to get a fecal transplant. He was willing to fly across the country but he couldn’t find a doctor willing to help. So he asked his roommate and they did the transplant in their apartment.
He was willing to fly across the country but he couldn’t find a doctor willing to help.
Smith, who studies the microbiome, heard his friend’s saga and decided something needed to change. He partnered with a few other graduate students and took over a little corner of his research lab. Soon, the first national stool bank was born.
They started screening possible donors, giving them a huge battery of tests. Dr. Edmond says it’s far more thorough than an individual doctor could do: the investment is worthwhile when these donors can make donations day after day.
“What we do is — maybe you can think of it like the TSA pre-screening situation," Smith said. "We invest a lot of energy in screening someone carefully and we know they are safe. And then we can use them again and again without have to reinvest all this energy in re-screening them.”
Most of their donors are folks that work near the stool bank, often at MIT. That way, when they are ready to make a donation they can just swing by and deposit it.
OpenBiome uses an age-old strategy to draw donors in frequently. Remember that trophy? It’s all a big competition. The 'Most Generous Contribution' award goes to the donor who produces the single largest stool.
“Some of the samples are enormous and can save 10 patients," Smith said.
Once OpenBiome has the donated stool, they prepare it and freeze it in those two hefty refrigerators. Now, when a doctor calls up and needs one of those bottles, they can just ship it over. They say the goal is to make it easy for doctors. So easy that doctors will start doing the transplant.
Dr. Edmond recently switched over to using OpenBiome material.
"It's much simpler using OpenBiome’s product because I don’t have to do any donor screening," he said. "The patient doesn’t have to identify the donor. They don’t have to pay for any donor testing. And I don’t have to have a tech involved because everything comes ready to go. And it cuts my time for each procedure from three hours down to one hour.”
OpenBiome charges about $250 for 250ml of stool — about a cup. Dr. Edmond says it’s the deal of a century.
The FDA Saga
But not so fast. The Food and Drug Administration must have something to say about this, right?
Yes, they are in the process of figuring out what they want to say. Back in May of 2013, the FDA hosted a workshop about fecal transplants. According to reports from the workshop, the agency was concerned that fecal transplants could cause infections if done improperly, and were being advertised for "unproven purposes." Plus, the transplants might have long-term risks that we don’t yet know about.
So, they decided to treat fecal transplants like an experimental drug. This meant that doctors would have to get approval before doing each procedure. Dr. Edmond says it’s a long and laborious application process. Some doctors say that hurdle would stop them from doing the procedure altogether — it just wouldn’t be worth it to them.
But at the FDA workshop, there was a patient outcry. Literally, one patient crying.
It was Catherine Duff. She was apparently the only patient present. And after one day of fuming silently, she decided to speak up.
At the FDA workshop there was a patient outcry. Literally, one patient crying.
“And I have a terrible fear of public speaking," she said. "But I was more mad than I was afraid. So I got up and I gave as much of the talk as I could. I was crying and I couldn’t really see my iPad very well. My voice was shaking. I felt like I was going to pass out.”
She told the FDA its decision was going to deter doctors and kill patients.
“At the end of it, I got a standing ovation and about half the people in the room were crying. And after that the meeting took on a whole different tone.”
The attendees were still in there discussing what to do when the lights were turned out and the building was closed for the night, Duff recalls.
In the end, the FDA changed its stance. It decided to “exercise enforcement discretion.” Essentially, the FDA was willing to turn a blind eye and let doctors perform fecal transplants on C. diff patients. This effectively left the field highly unregulated: there were no designated protocol to follow, screening tests to perform, data to gather.
That’s where things stood when OpenBiome started shipping out those bottles of stool this October.
But late last week, the FDA quietly posted a potentially pivotal change on its website. It’s a draft of new guidelines. And it says something that shocked Dr. Edmond.
“[The] FDA intends to exercise this discretion provided that….[the donated stool] is obtained from a donor known to either the patient or to the licensed health care provider treating the patient.”
That’s a problem for OpenBiome, which makes sure its donors remain anonymous.
Duff says the requirement to "know" the donor leaves her baffled: “Does that mean you have to have sat down and had a meal together? Does that mean you’re Facebook friends?"
When I asked the FDA Duff's question, they responded in writing simply saying "there are no specific criteria defined for 'known to'."
Dr. Edmond believes there needs to be FDA oversight but he is concerned about this proposal. He says the best analogy is to blood banks, which actively avoid letting donors give to recipients they know.
These donors "are less likely to be honest about risk factors they may have for infectious diseases," Dr. Edmond says. "And they feel compelled to do it. If your grandmother says ‘I need your stool or your blood.’ You may not want to say, ‘Maybe I am not the best donor because I have risky sexual practices’.”
The FDA did not respond directly to this concern. However, they wrote that the stool bank's material could still be used. But the doctors would have to go through that laborious application process - the one that initially caused the patient outcry. They say there might be a way to shorten the application for doctors who use material from a stool bank but that's still being worked out.
The FDA is trying to balance the need for C. diff patients with their own need to oversee the process and gather data.
"The draft guidance document is intended to accommodate those individual sick patients, while assuring that FMT [Fecal Microbiota Transplantation] product that is manufactured from the stool of a donor who is not known by either the patient or the licensed health care provider would be subject to appropriate manufacturing and regulatory controls, and where its safety and effectiveness could be studied...the science available at this point dictates that FMT is at the stage where it is an experimental procedure and its safety is not assured."
Now remember, the FDA does not have specific manufacturing or regulatory guidelines. They simply want to check over those self-designed screenings - but only if the donation is anonymous. The hope is that this would give them enough data to start moving fecal transplants out of the 'experimental drug' category. Plus, it might prevent lackadaisical stool banks from springing up.
Right now, the FDA’s is soliciting feedback on the draft. So nobody knows exactly what will happen. Sometimes draft guidelines never go into place. Sometimes they do, but only years later.
If the guidelines do go into effect as currently worded, Dr. Edmond is not sure he will continue performing fecal transplants.
"If it’s going to be three hours again for me - because I got it down to one hour when I moved to OpenBiome - I have to really rethink whether I’m going to do it or not,” he said.
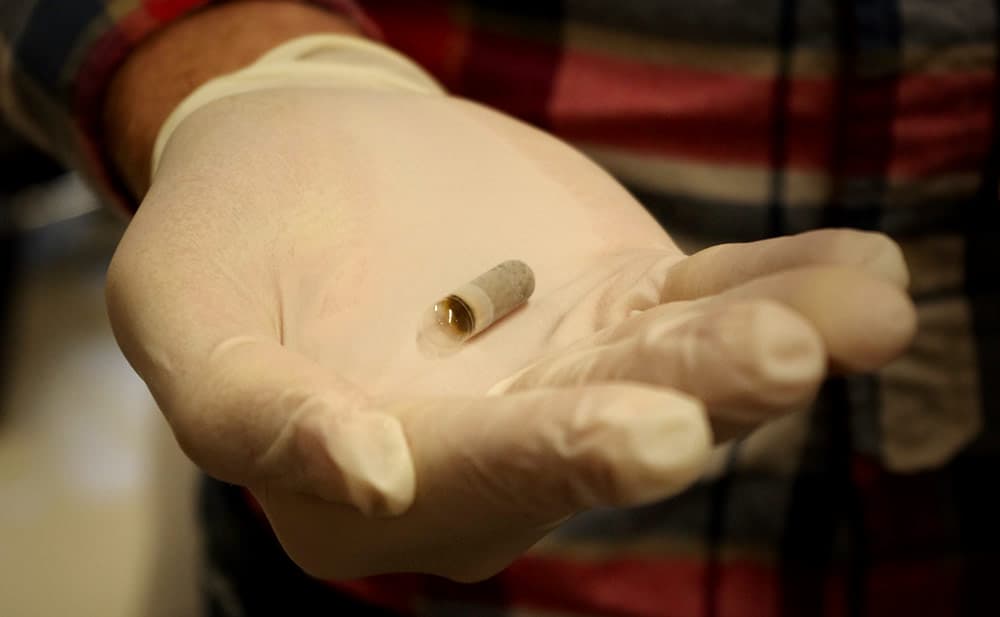
The Poop Pill Of The Future
Even those who are fighting hardest for the stool bank will say fecal transplants are not the way of the future. They are just a transitional solution.
To the side of the fume hood and some specimen collection bins, there are empty little capsules. Smith believes those might hold the future.
When he is not working on the stool bank, Smith is trying to develop what he calls The Poop Pill. He envisions C. diff patients drinking a glass of water and simply gulping down a pill. Inside that gelatin capsule would be strains of happy bacteria.
The goal is to create a pill that “when it’s in the gut will dissolve and break open and release its bounty of bacteria.”
It’s a bit challenging technically, to keep the bacteria from breaking down the pill prematurely. They are looking at various coatings and perhaps making an inner capsule and an outer capsule.
Smith has come up with a pill that he thinks will work. It's now going through a university review process. He says if everything proceeds smoothly, the first patient will try it out in the next month or two.
He has even picked out the first poop pill patient. She is a quadruple amputee with C. diff. She’s not a good candidate for a fecal transplant, but her doctor thinks this pill might be perfect, Smith says.
At the same time, there are other companies experimenting with creating synthetic poop. These pill based solutions might make doctors more comfortable. And the goal is to allow doctors to treat C. diff earlier, instead of turning to fecal transplants as a last resort. That, in turn, might make C. diff less prevalent in hospitals.
Smith doesn’t know exactly what the future holds. But as he looks at his refrigerators full of stool donations, he says, “I don’t think this is going to be going on for that much longer. I think in a few years this will be some crazy relic of the past and then we will be on to better solutions.”
Updated with FDA response on 3/13/2014.