Advertisement
The Promise And Price Of New Addiction Treatment Implant
ResumeAmid a raging opioid epidemic, there's a plea for more treatment options. The Food and Drug Administration expects to have a decision on one by May 27.
It's an implant. Four rods, each about the size of a match stick, inserted in the upper arm. This new device, called Probuphine, delivers a continuous dose of an existing drug, buprenorphine, but with better results, says implant maker Braeburn Pharmaceuticals.
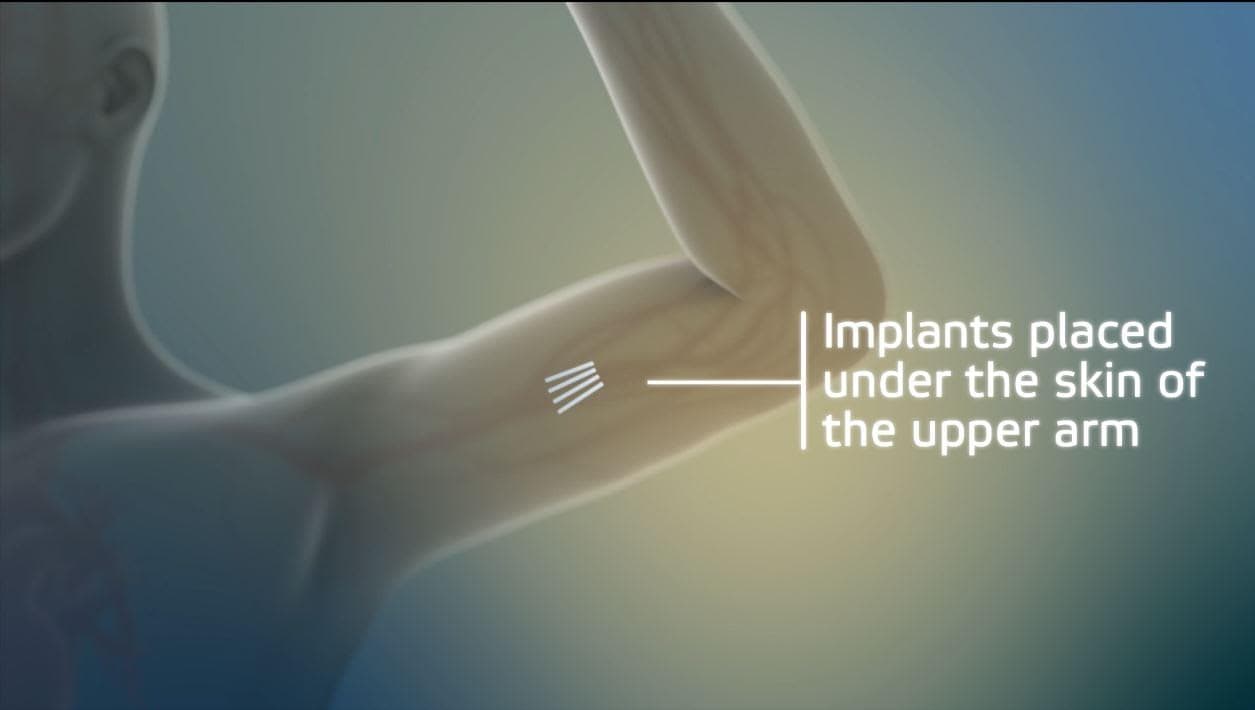
In clinical trials, 88 percent of patients with the implants abstained from opioids, as compared to 72 percent of those taking buprenorphine as a daily pill. (Buprenorphine is commonly referred to by its brand name, Suboxone.)
"I felt completely normal all the time," said Dave, a paramedic in a small town outside Boston who was on the implant during a clinical trial. He does not want his last name made public so that coworkers won't find out he is addicted to opioid pain pills.
Dave, 47, has been in recovery for four years with the help of buprenorphine. Dave said he prefers the implant to the pills for several reasons. With the pills he would sometimes feel the drug wear off. He worried about his 2-year-old granddaughter getting into the bottle. And sometimes Dave would just forget to take his medication, which he’s supposed to do in the morning, 15 minutes before he has anything to eat or drink.
"With the implant you didn’t have to worry about that, you just, it was just there and you felt good all the time," Dave said.
There's a second reason the manufacturer says its implants are better than pills.
"Buprenorphine is the third most confiscated opioid by the DEA, so there’s certainly diversion going on," said Braeburn Pharmaceuticals CEO Behshad Sheldon, referring to illegal sales of the prescribed drug.
But not with the implants, according to Sheldon. She says no one tried to remove their implant during trials so they could sell the drug inside.
An FDA advisory committee reviewed the implant in January and recommended approval.
Dr. Barbara Herbert, president of the Massachusetts Society of Addiction Medicine, is one of many providers waiting for the agency's decision.
"Anything that might help people beat their opioid addiction is a good idea," Herbert said.
But she has reservations.
Probuphine is only available in one dose, the equivalent of 8 milligrams per day. You can’t cut a rod in half as you might a pill or adjust the dose. Herbert said that could be a problem.
"We don’t want to overshoot and give people more buprenorphine than they need, because it makes them somnolent. And for some people they’re going to need more," Herbert warned, which takes patients back to the challenge of remembering to take a daily pill. The company said the 8 milligram dose will be effective for a large number of addiction patients.
Herbert is also concerned about price. Sheldon said the cost of the implants will be competitive with other treatments, including Vivitrol, a shot that is $1,000 a month. Herbert said at that rate, providers may be forced to turn patients away or cut back on other services. She questions whether the price will be justified.
"High profits in the middle of this epidemic are really unconscionable," Herbert said.
Sheldon said there will be rebates and assistance with co-pays to make sure patients can get the implant. The device may mean fewer patients sharing needles and contracting infectious diseases which Sheldon says will save money for doctors and health insurers.
"If they don't realize those savings we're happy to rebate them even further," Sheldon said.
Even at close to a $1,000 a month, demand for the implants, if approved, is expected to be high as more and more Americans struggle with an opioid addiction and more physicians approach addiction as a chronic disease.
"With the implant you didn’t have to worry about that, you just, it was just there and you felt good all the time."
Dave, paramedic in recovery
Dave, the paramedic who is in recovery, said he's thought about trying to wean himself off the treatment drugs.
"But then, the more I think about it, it scares the hell out of me, cause I’m scared of going backwards. I honestly don’t know what would happen," he said.
The company will need to train doctors on how to insert the implant, if approved. Sheldon said she doesn't know yet if reinserting the device into the same opening in the arm will work or if doctors would have to create a new scar for each six-month replacement.
And then there's the problem of patient limits. Right now physicians cannot treat more than 100 patients with buprenorphine. The Obama administration wants to raise that cap, and Congress is considering legislation that would do so. Sheldon said one bill would exempt buprenorphine implants from the 100-patient limit, which could effectively increase the cap as well.
This article was originally published on May 19, 2016.
