Advertisement
FDA Finds Safety Issues at 30 Compounding Pharmacies
Resume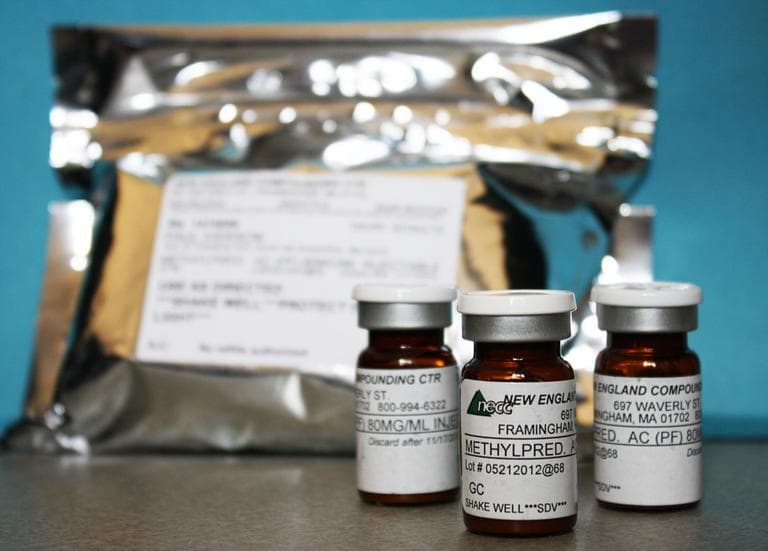
Unidentified "black particles" in vials of medicine. Rust and mold in rooms meant to be sterile. Inadequate testing for microbes. Tears in gloves worn by staff working on medicine.
Those are among the potentially dangerous safety problems the Food and Drug Administration has found at what are called "compounding pharmacies" during inspections conducted between February and April, according to The Washington Post.
Compounding pharmacies can mass produce medications but they are regulated under different laws from drug manufacturers.
Filthy conditions at a compounding pharmacy in Massachusetts were responsible for a fungal meningitis outbreak that killed 53 people across the U.S. and sickened hundreds of others.
This is the first set of FDA inspections at compounding pharmacies since that outbreak.
The agency is still analyzing the data, but the findings have already led to recalls and temporary suspensions of drug production.
- Washington Post: FDA finds widespread safety issues at compounding pharmacies
Guest:
- Lena H. Sun, health reporter for The Washington Post.
This segment aired on April 12, 2013.