Advertisement
How Researchers Are Putting The Body's Own Army To Work To Fight Cancer
Resume
This story is part of our "This Moment In Cancer" series.
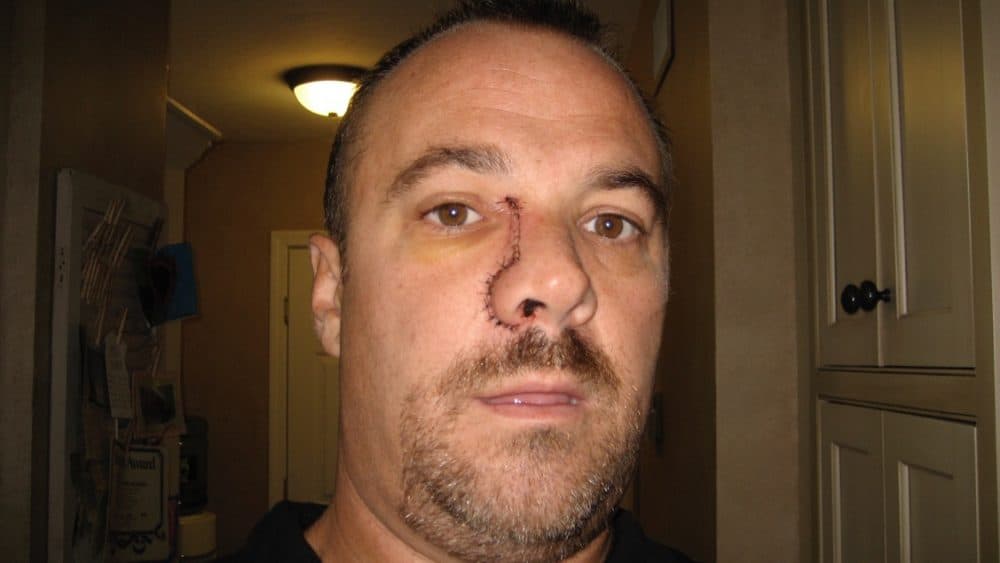
Richard Murphy’s sinuses were so stuffy that for months he couldn’t sleep more than two hours a night. Doctors kept blaming Murphy's allergies and telling him he needed shots. But this didn’t feel like allergies.
Murphy, of Marshfield, finally sought out the ear, nose and throat doctor who had treated his three kids. “I feel like there's a golf ball in my nose,” Murphy told him. The specialist took out a tool, looked up Murphy’s nose, and agreed: “There's a golf ball up your nose.”
That "golf ball" was soon diagnosed as melanoma -- a form of cancer that, if it spreads, is considered among the deadliest of tumors.
Murphy had the tumor removed and endured 10 weeks of radiation, commuting between Dana-Farber Cancer Institute and his family’s small business in Rhode Island.
"At the end of the 10 weeks I was pretty tired, but my wife and I sat there and were like, 'All right, I think we just dodged a bullet here,' " Murphy says. “You’re feeling pretty confident when you don't know any better."
Radiation, chemotherapy and surgery are great at shrinking or cutting out tumors. But these approaches can miss cancer cells that then seed new tumors.
That’s why researchers are increasingly pinning their hopes on a new treatment strategy: using the immune system to hunt down and kill cancer cells wherever they are in the body.
“There's no doubt that immunotherapy is the new yellow shiny toy,” says Greg Simon, the former head of the Cancer Moonshot Task Force set up by former Vice President Joe Biden. “The question is: Is it going to work in enough people to justify the billions that will be invested in it?”
“There's no doubt that immunotherapy is the new yellow shiny toy. The question is: Is it going to work in enough people to justify the billions that will be invested in it?”
Greg Simon, former head of the Cancer Moonshot Task Force
'Coley's Toxins'
More than a century ago, a New York bone cancer surgeon named William Coley noticed that some of his patients recovered spontaneously from their cancer after they caught infections.
The idea made sense: The immune system is designed to hunt down foreign cells anywhere in the body, so it seems like the perfect tool to seek out and destroy cancer cells.
Coley began infecting patients with a cocktail of bacteria that came to be called "Coley’s Toxins." He had some successes, but his approach fell out of favor when radiation treatments and chemotherapy came along.
Over the second half of the 20th century, researchers occasionally tried again to get the immune system to fight cancer. But nearly every time they either failed to get it to recognize cancer cells or revved the immune system up too much and sickened or even killed patients.
There’s a delicate balance between encouraging the immune system to fight cancer and pushing it into overdrive, says Tyler Jacks, director of the Koch Institute for Integrative Cancer Research at MIT.
"We don't want to stimulate the immune system to fight normal cells and tissues — it's really a fine line," he says. "The body has many control mechanisms to keep the immune system focused on its targets to avoid autoimmunity and instead just fight cancer."
Releasing The Brakes – Without Causing An Accident
About 20 years ago, researchers began to realize that cancer somehow stopped the immune system from hunting down and killing tumor cells -- it put brakes on the immune system. Scientists began testing drugs, called checkpoint blockades, that could metaphorically lift cancer’s foot from the pedal.
The first cancer to prove the effectiveness of these checkpoint blockades was metastatic melanoma, the disease that Richard Murphy thought he had dodged. Unfortunately, by mid-2011, three years after his “golf ball” was removed, tumors were popping up throughout Murphy’s body.
Most doctors would have told a patient with scans like Murphy’s to get his affairs in order and say his goodbyes. Instead, his oncologist at Dana-Farber put Murphy on the first of these checkpoint blockade drugs, called ipilimumab (now sold as Yervoy).
At first, Murphy’s results were dramatic. His tumors quickly shrunk by 25 percent. He thought he’d dodged another bullet.
But just a few months later it became clear that the drug wasn't working anymore. His doctor took him off of it and told Murphy he didn’t have any other viable treatment options to offer.
“When you're in a treatment you know what you need to do, you've got a plan. But when you're not in a treatment ... There was nothing worse than being off the treatment.”
Richard Murphy
Those months, Murphy said, were the toughest of his whole cancer battle. “When you're in a treatment you know what you need to do, you've got a plan,” he says. “But when you're not in a treatment ... There was nothing worse than being off the treatment.”
Then, the next generation of checkpoint blockades started clinical trials. Murphy’s doctor managed to get him into one.
He was doing well on the drug, called pembrolizumab (now sold as Keytruda), and was scheduled for his fifth monthly dose on the day his kids finished their 2012 school year. He told his wife he could handle the treatment by himself and would be home in time to pick the kids up from the school bus and celebrate the start of summer.
Unfortunately, that’s not how his day turned out. “People plan, God laughs — is that the Yiddish term?” Murphy says, chuckling at the memory.
Although he felt fine, blood tests showed Murphy’s kidneys were failing. His oncologist told him to skip the drug treatment, walk down the street to Brigham and Women's Hospital, and check himself in.
That night, lying in his hospital bed, Murphy’s doctor gave him good and bad news: The checkpoint inhibitor seemed to be shrinking his tumors. But the drug company was kicking him out of the trial, worried that if he kept taking the drug he’d suffer worse than kidney failure.
"So let me get this straight: The drug's working, but you're yanking me off it?" Murphy asked his doctor with a mixture of panic and annoyance.
He spent the next several months without any treatment, soothing his anxiety by running races to raise money for Dana-Farber. As he prepped for a triathlon in the summer heat, he suddenly gained 25 pounds in just two weeks.
Tests showed that his thyroid had quit working. Probably a side effect of the cancer therapy, his immune system had gone into overdrive and attacked his thyroid. Many immunotherapy patients have reported similar autoimmune side effects. Some have suffered worse.
A daily pill quickly brought Murphy’s thyroid issues under control.
And his next checkup with his oncologist was nothing short of miraculous: Though he hadn't taken a drug in months, and should have been dying, every one of his tumors had completely disappeared. He didn't need treatment anymore, because there was nothing to treat, his doctor told him.
“That's where we've been for four years,” Murphy says, with a grin. “It's been a crazy roller coaster ride. I’m the luckiest person in the world.”
On The Cusp Of A Revolution
Murphy, now a 51-year-old real estate agent, remains an exception — one of the luckiest of those unfortunate enough to get a particularly lethal form of cancer.
Checkpoint blockades are now approved to treat lung, bladder, kidney, and head and neck cancer, as well as melanoma, and there are more and more seemingly miraculous results like Murphy’s.
Still, at best, only 10 to 40 percent of patients benefit from checkpoint blockades, depending on which drug and which cancer type.
"There are a large number of patients who are feeling disappointed and frustrated and left out. We have not solved this,” says Dana-Farber lung cancer specialist Geoffrey Oxnard.
Oxnard tells patients he can't really predict how they will respond to the new drugs. “Some patients do great, some patients just get sick, and most patients are somewhere in the middle," he says.
But there’s a hopefulness now, Oxnard says, that he wasn’t able to offer even a few years ago. “There is a chance we can make this disease very livable and could allow you to live with this metastatic cancer for years,” he tells his patients.
Other forms of immunotherapy are being developed in addition to checkpoint blockades.
Several pharmaceutical companies are hoping to win federal approval this year to market a type of immunotherapy called CAR-T, which creates individual treatments for patients, re-engineering their immune system’s T cells to fight cancer.
These CAR-T therapies have shown particular promise against blood cancers, triggering remissions in more than 80 percent of patients with some types of blood cancer. But the treatment is very difficult, pushing many patients to the brink of death. It can lead to lifelong side effects and is expected to be extremely expensive.
And there are hundreds of clinical trials underway around the world testing checkpoint blockades in combination with other treatment approaches. Where one checkpoint blockade might help only a fraction of patients, Jacks, of MIT's Koch Institute, says many more patients might be helped by adding other approaches.
Such an add-on could be another immunotherapy, Jacks says. “It could be chemotherapy, it could be radiation therapy, it could be so-called targeted therapies that lead to cancer cell death.”
Of course, combining therapies also adds to the cost of treatment, with many of these new approaches running $100,000 or more each.
Just Happy To Be Around
In Murphy's case, the one-two punch of the two checkpoint blockade drugs that became available just as he needed them might explain why he's had such an impressive result.
Whatever gets the credit, Murphy is just happy to be around. During his cancer treatment, whenever he felt low, Murphy says he'd focus on living long enough to see the youngest of his three kids, his daughter Mackenzie, graduate from elementary school.
“During the bad time, you get certain dates in your mind,” he says.
Mackenzie walked across the balloon-covered stage last June, and her teary-eyed dad was there to cheer her on.
“I’m forever grateful,” he says.
Do you have a question about cancer research, treatment or care that'd you like us to investigate? Ask it here.
This segment aired on January 31, 2017.
