Advertisement
A Drug Was Made For Just One Child, Raising Hopes About Future Of Tailored Medicine
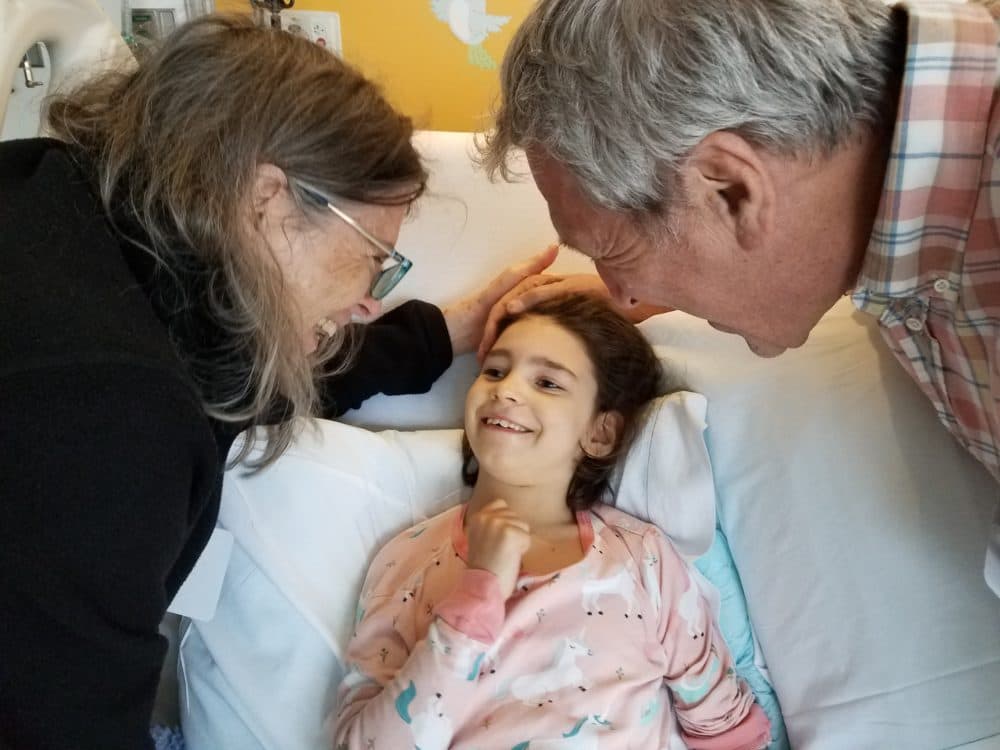
Julia Vitarello remembers the moment she realized that her daughter, Mila, was getting a drug designed specifically for her, one that might save her life.
“Dr. Yu brought me down the hallway and opened a refrigerator and said, ‘Look.’ He pulled a tiny little glass bottle out, and it said, ‘milasen,’ ” Vitarello says. “I remember I just started crying. Suddenly, now Mila was receiving this drug called milasen.”
Milasen is thought to be the first example of a treatment created for just one person that's likely to work only for her. In a paper published Wednesday in the New England Journal of Medicine, scientists reported the drug reduced or halted several symptoms of a disease that is always fatal, usually killing patients by the time they reach their late teens.
The apparent success of milasen is stunning proof of how these designer genetic drugs, known as antisense oligonucleotides, can be personalized for individuals, researchers say.
“This is a kind of fantasy that we all had at the beginning of this field more than 30 years ago,” says Dr. Cy Stein, an oncologist at City of Hope Medical Center in California who studies these drugs and was not involved in creating milasen. “Reading this paper is like reading [science-fiction author] Jules Verne. It’s a futuristic experiment.”
Searching For Mila's Broken Genes
Drugs like milasen can only work for genetic diseases that arise from a certain kind of mutation that causes genes — and the proteins they code for — to be incorrectly assembled. Certain types of cystic fibrosis, ALS, spinal muscular atrophy and, in the case of 8-year-old Mila Makovec, Batten disease, fall into this category.
Batten disease is ultra-rare, affecting only 2 to 4 out of every 100,000 children in the U.S. The first symptoms strike around age 3. Children who have the disease must inherit two broken copies of a gene — one from each parent — that’s critical to neural cells' ability to process waste.
“When this gene is mutated, this system doesn’t work," says Dr. Timothy Yu, an assistant professor at Harvard Medical School and physician at Boston Children's Hospital who led the team that created milasen. "The cell gradually gets sick, gradually stops working and gradually dies.”

When Mila was 2 years old, she loved chatting, singing and hiking with her mother. By the time she was 3, Mila would scramble up climbing walls 30 feet in the air and march through the forested hills near her family's home in Boulder, Colorado.
“She had tiny little hiking boots, and she would hike through the snow and the rain,” Vitarello says.
Then Mila’s abilities began slipping.
"She just seemed to be changing. She started tripping. She started stepping on her toys,” Vitarello says.
Their house turned into a scrapyard of dented, demolished toys and tattered books, shredded by Mila’s feet running over them.
“I realized later that her vision was going," Vitarello says. "She was not able to make as much sense of the world around her."
When Mila was just turning 6, nothing had seemed to improve her symptoms. Finally, doctors at Children’s Hospital Colorado diagnosed Mila with Batten disease. Vitarello learned her daughter was going to die, probably before she was 20.
Advertisement
“The best way to describe [how I felt] was I could not be around the world," Vitarello says. "I could not go outside of my house. I could not see other children that were Mila’s friends that were skipping and jumping and playing soccer."
Vitarello started a nonprofit called Mila’s Miracle Foundation, hoping to raise money for a gene therapy that might prolong her daughter’s life. But doctors could only identify one of Mila’s Batten disease mutations. Unless they could find the second mutation, there was no way to develop a treatment for her. Vitarello turned to social media to see if anyone could put her in touch with a researcher who could help find Mila’s broken genes.
Yu says he and his wife found that message on Facebook. Yu’s lab has worked on genome sequencing for 10 years and had expertise in finding rare mutations. “It seemed we could solve the puzzle for the family.”
One Drug For One Patient
Two months later, Yu and his team in Boston had found Mila’s second Batten mutation.
“This was a really unusual case,” he says. Genes are written like a sentence explaining how a protein should be put together. But in Mila’s case, an extra piece of genetic code was thrown into the middle of that sentence, Yu says.
“That instruction is nonsensical," he explains. "When the cell reads into it, it just sees this garbled message and stops making protein.”
Yu had seen something like this before. The company Ionis had just created a drug called Spinraza that designed a bit of synthetic DNA to fix a similar mutation for spinal muscular atrophy. Yu thought if Spinraza could get cells to read a gene correctly that had the same type of mutation, maybe he could create a similar drug tailored to Mila’s gene. He called Vitarello with the news.
“That call ended up being the call that changed my life,” Vitarello says. “He — thank God — confirmed that my son, Azlan, was not going to [get Batten disease]. Then he paused, and he said, ‘You know, I have an idea of how I might be able to help Mila.’ ”
The drug Yu and a team of over 40 researchers across the country created is a bit of genetic code uniquely tailored to Mila’s genetic mutation.
“It acts as a molecular patch,” Yu says. The custom genetic drug simply covers up the messed up part of the gene, like crossing out nonsense.
“To our great happiness, it allowed the gene to be spliced back together in the proper way, ” Yu says.
Working Against Time
The scientists finished the design, manufacturing and testing of Milasen just 11 months after Yu and Vitarello first spoke — a process that normally takes drug companies several years. The FDA gave the team approval to administer the drug in January 2018, and doctors infused the treatment into Mila’s spine at the end of that month.
By that time, Mila had lost the ability to see, speak most words and walk without assistance. She was starting to slump over when sitting and was having seizures so violent that she would bruise her legs against tables and chairs. But within six months, Mila seemed to be doing better.
“We never saw these really severe seizures again,” Vitarello says.
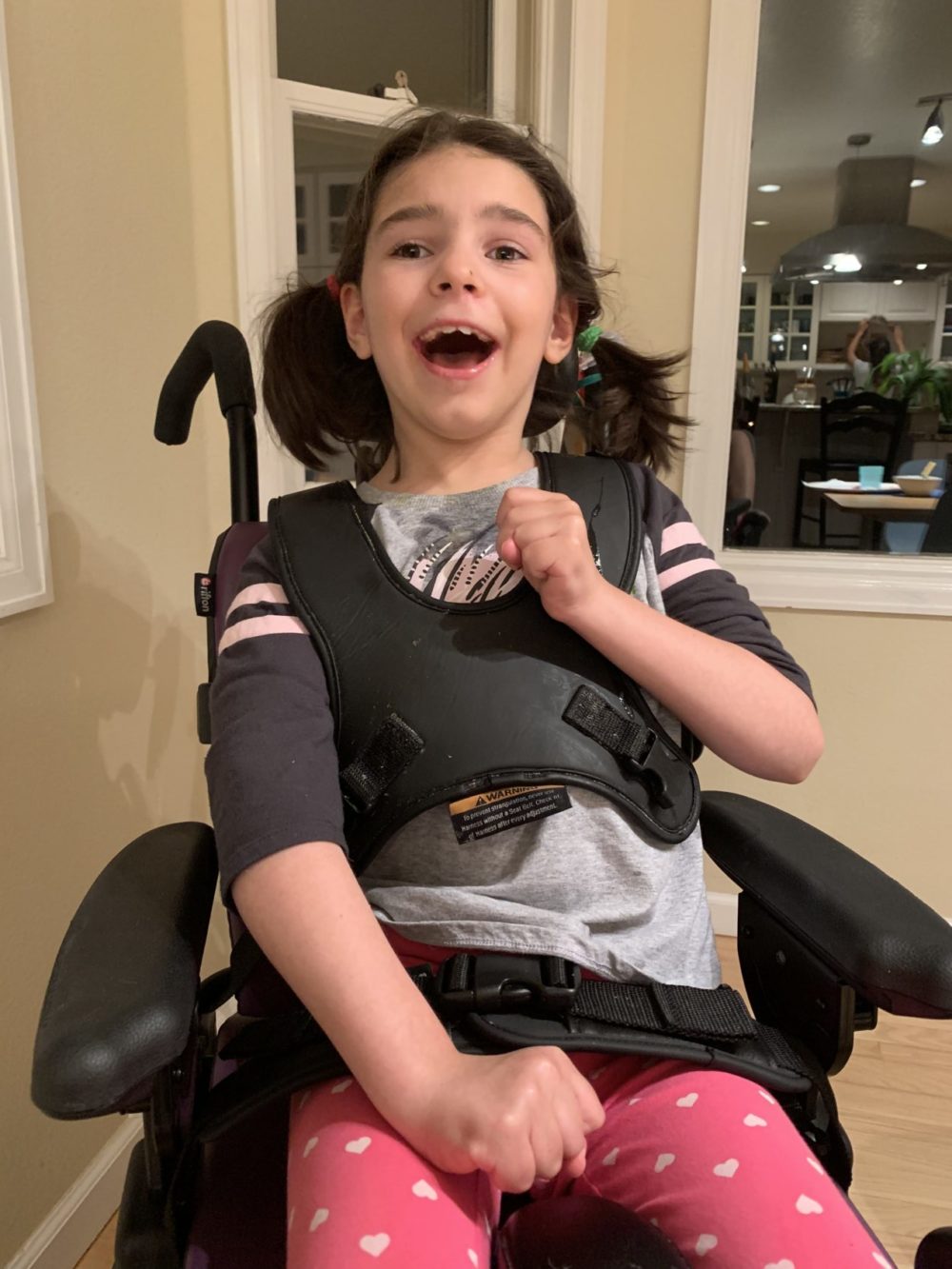
In their paper, Yu and his team reported that Mila went from having 15 to 30 seizures per day, each lasting roughly 2 minutes, to 0 to 15 seizures per day, lasting just a few seconds. Now, Vitarello says, it’s difficult to even notice them.
Milasen is far from a cure, however, and Vitarello says while Mila is getting better in some ways, she seems to be getting worse in others. She no longer responds as well or as quickly to her favorite songs and stories, for instance.
“[Milasen] is not an optimal drug,” says Dr. Don Cleveland, a geneticist at the University of California, San Diego, who was not involved with the research. His lab was the first to create a successful antisense oligonucleotide drug.
There’s still a lot of research to be done to understand how to best administer milasen to Mila, and whether it can be tweaked to be more effective. “But it’s quite good,” Cleveland adds.
For researchers like Cleveland, Mila’s story is proof that it’s possible to tailor genetic therapies to single individuals.
“It is sort of amazing,” he says. “This paper is demonstrating you really can use designer DNA drugs for these individualized therapies. They showed that they could do it in a short time frame. It’s really uplifting.”
Ethical Questions
The hope, Cleveland says, is that researchers can use the same steps Yu took to create milasen to create custom drugs for mutations that cause thousands of other rare diseases. Yu estimates there may be 1.4 million people in the world with rare and fatal neurodegenerative disorders that might benefit from this approach.
But the promise of designer genetic drugs for individuals also raises several ethical questions. Senior Food and Drug Administration officials Janet Woodcock and Peter Marks argue that in cases where there’s only one or two patients, knowing if a drug will even work or worse — cause further harm — is difficult.
In an editorial published Wednesday in the New England Journal of Medicine, Woodcock and Marks ask: “What type of evidence is needed before exposing a human to a new drug? How should the urgency of the patient’s situation, or the number of people who could ultimately be treated affect the decision-making process?”
There's also the question of price. Neither Yu nor Vitarello have disclosed how much it cost to create milasen. The project was funded by several sources, including Mila's Miracle Foundation, research grants and Boston Children's Hospital. Clearly not every patient with a rare disorder will have the ability to raise that kind of funding.
Mila will die without regular infusions of her drug. Even with it, she is unlikely to regain certain abilities, like her sight.
“But she’s still herself,” Vitarello says. “She’s still smiling and laughing. She’s listening. She loves music. She’s still there.”
Vitarello doesn’t know what will happen to Mila in the future, but she says her story means scientists and regulators are now laying out the ethical, regulatory and scientific path for more people like her in the future.
“I think that’s Mila’s gift to the world,” Vitarello says.
This article was originally published on October 11, 2019.
This segment aired on October 11, 2019.
