Advertisement
Commentary
Rewriting Our Genes Is Easier Than Ever. That Doesn't Mean We Should Do It
Gene-editing technologies have huge potential to alleviate human suffering. But, like all very powerful technologies, they also carry enormous risks if used improperly.
In November 2018, a team of scientists in China led by Dr. He Jiankui revealed shocking news at a conference: he’d used CRISPR-Cas9 (often referred to as just CRISPR) to edit the genes of three embryos. Two of the embryos were successfully implanted in a surrogate, resulting in twin girls. Now known only as Nana and Lulu — their identities protected in scientific version of the witness protection program — Dr. He said he’d used CRISPR to immunize the embryos to HIV. But he’d acted against worldwide guidelines and regulations to do so. Those regulations prohibited “germline” edits, or genetic edits that are heritable to the edited organism’s future offspring. (Dr. He and his collaborators were recently sentenced by a court in Shenzhen to three years in prison for conducting "illegal medical practices.")
Let’s back up.
Gene editing is what it sounds like: modifying an organism’s genes. The technology has a massive range of applications, and those applications carry different degrees of risk, depending on the kinds of cells edited.
Maybe you remember the distinction of somatic versus non-somatic cells from biology? If not, here’s a refresher: it’s the difference between cells involved directly in reproduction (non-somatic) and cells not involved in reproduction (somatic). Most of your cells are somatic: your eyes, your lungs, your heart. For non-somatic, think sperm, eggs, embryos, stem cells: the cells directly used to create offspring. The difference is relevant because genetic modifications to reproductive (non-somatic) cells get passed on to the descendants of those organisms.
In essence, if you edit an organism’s somatic cells (kidneys, blood, etc), that edit dies when the organism does. If you edit non-somatic cells, and the organism reproduces, its offspring’s reproductive cells will have the same edit, which will be passed on and on, as long as the edited organism’s genetic line keeps reproducing.
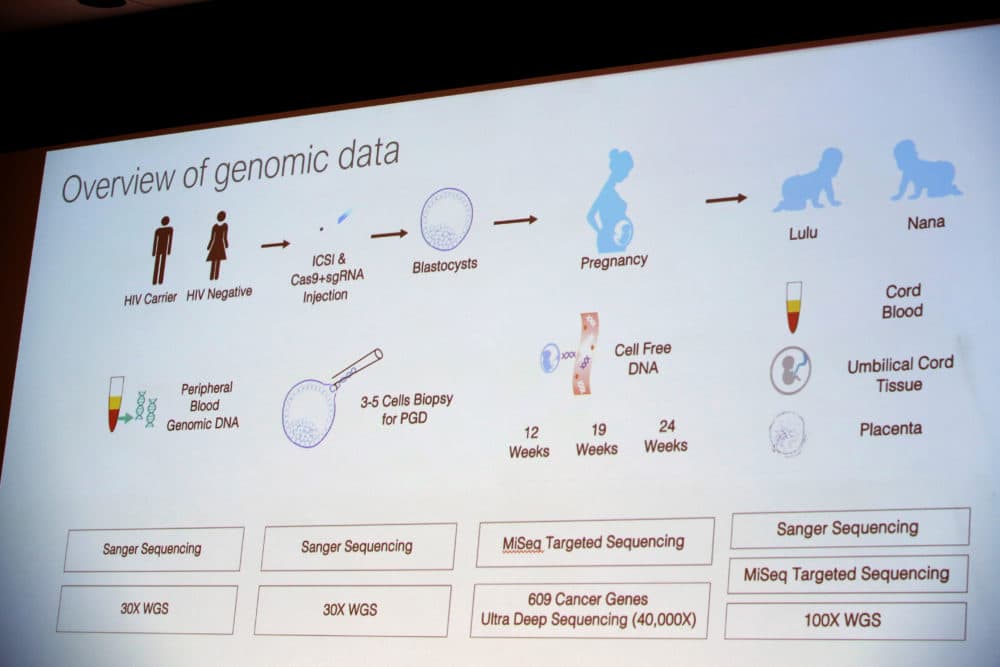
The results of Dr. He's genetic edits to embryos demonstrated the reasons for the ban on human germline editing: he might have inadvertently made unintended edits to the embryos’ other genes. His rogue experiment’s consequences might have significant adverse effects for Nana and Lulu, whose germline, or non-somatic genes, he edited.
And CRISPR’s no longer the only gene-editing game in town. There was news last fall of a new gene-editing technology called "Prime-Editing." The developers claim it’s more precise than CRISPR.
The majority of the scientific community continues to agree on a moratorium on human germline editing. But that word, “moratorium,” has a temporary connotation, and some scientists are likely to agitate for human germline editing with this new technology, despite the widely acknowledged disaster of Dr. He's first foray. Some might argue that this more precise method of gene editing would be safe to use for human germline edits.
That argument pushes us towards a risk we shouldn’t take. “Prime-Editing” is more precise than CRISPR, but that doesn’t make it “safe” for this purpose. If germline gene editing goes wrong, there’s no ethically sound way to stop the resulting domino effect.
... there is no precedent -- nor should there be -- for preventing a person who hasn't even been born yet from reproducing as an adult
Gone wrong, germline gene editing has the potential to do widespread damage. Consider the recent finding that all living human beings descended from one woman who lived in the area we now call Botswana. Scientists have referred to this common ancestor as “Mitochondrial Eve.” Take her as an example — an extreme, but real, example — of the potential reach of one individual’s genes.
Editing an embryo’s germline genes means that you’re altering the genetic code of that person, for life — and, if that individual has a baby, they may pass on those altered genes to that baby.
If Nana or Lulu have children, they’ll pass on the germline edits Dr. He made — both the intended and the unintended. When they come of age, Nana and Lulu will have to have a version of the “where babies come from?” talk that no human being has ever before experienced, or should have to.
The only way to prevent the future transmission of germline edits is to prevent the person whose genes have been edited from reproducing. Nana and Lulu couldn’t have possibly consented to that as a condition of the experiment, because they weren’t alive when the experiment was performed. Limiting their reproductive possibilities in that way would approach eugenics.

A more precise technology might be better, but it still isn’t perfectly precise.
Internal review boards, the ethics committees that review proposed scientific research, make many fraught calculations, but there is no precedent — nor should there be — for preventing a person who hasn't even been born yet from reproducing as an adult. It’s the internal review boards' job to contain possible problems, but they can't contain this problem without causing another.
The “if we can do it, we will” argument doesn’t hold with what today’s technology makes us capable of. We shouldn’t follow the hinge of every “if, then” to an unknown, potentially catastrophic outcome. We have to set some hard limits on what we’ll do with the technologies we develop.
This is one such example.
