Advertisement
Study: New Way To Hold Back Herpes, Keep Virus Latent
Chances are, you've got herpes, I've got herpes, we've all got herpes.
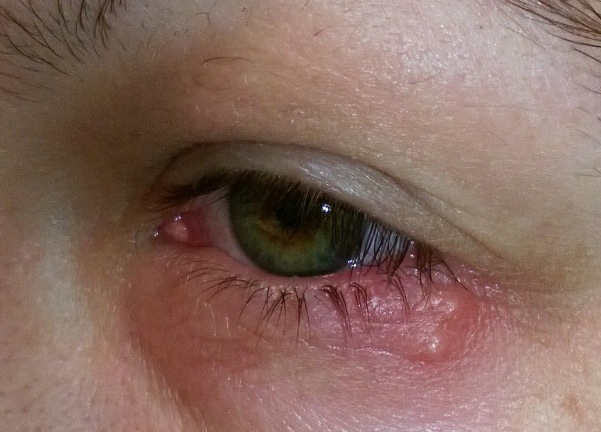
Studies find that by age 60, virtually all adults carry herpes simplex virus 1 — best known for seeping cold sores but also potentially blinding when it hits the eyes. Herpes simplex virus 2, the sexually transmitted disease, infects more than a quarter of people by their forties, the CDC says.
While anti-viral medications can help, there is no cure for herpes viruses. Their wily ways of going latent between recurrences, hiding out in viral reservoirs in our bodies, make them supremely hard to eradicate.
So it's welcome news that a study just out in the journal Science Translational Medicine describes a whole new strategy for beating down herpes viruses and keeping them down — at least in mice, rabbits and guinea pigs.
Far fewer of the mice died or had virus spreading throughout their bodies.
It's a tactic that researchers say may also hold promise for attacking HIV, another virus whose habit of hiding out makes it hard to kill, and the herpes zoster virus that causes excruciating shingles.
The new method hinges on epigenetics — specifically, protein "packages" that determine how genes are turned off and on.
For a herpes virus to go from a latent state to an active state, it needs to unpackage or unbundle its genes so they can be "turned on" and begin to replicate and spread. But, the researchers found, if they block an enzyme called LSD1, those genes tend to stay bundled up and inactive.
It's as if the viral DNA encoding the genes needed to reactivate the virus naturally carries a "Don't open me!" sign on it, says the paper's senior author, Dr. Thomas M. Kristie. The LSD1 enzyme can remove that sign. But block the enzyme and the "Don't open me!" sign stays up.
"The primary point of the paper is that you can epigenetically suppress a viral infection, and specifically, viruses that cause persistent infections," says Kristie, chief of the Molecular Genetics Section of the Laboratory of Viral Diseases at the National Institute of Allergy and Infectious Diseases.
To block that key LSD1 enzyme, the researchers used an existing antidepressant, called tranylcypromine. As it happens, tranylcypromine also blocks LSD1.
The antidepressant offers proof of concept, researchers say, but if the new method is eventually used on human patients, it's likely to be in the form of new drugs developed specifically to act on LSD1.
The paper reports that this new, epigenetic method appears effective at several stages of herpes infections:
• In mice used as a model for herpes transmission at birth, far fewer of the mice died or had virus spreading throughout their bodies.
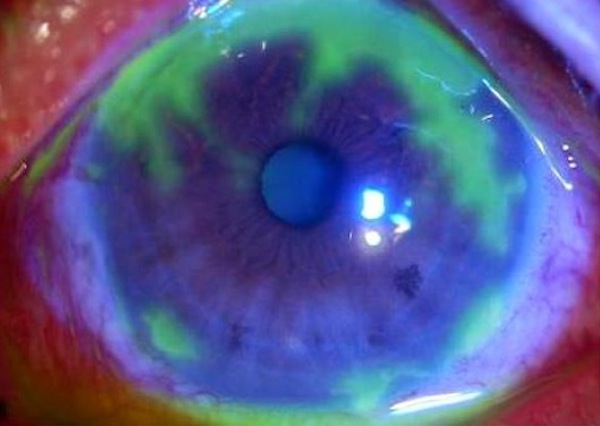
• Among rabbits infected with herpetic keratitis — the herpes infection of the eye — far fewer had recurrences and there was far less "shedding" of the virus, which is how it's usually transmitted.
"With herpetic keratitis, what happens is you get a small amount of the virus reactivating from latency, but that also causes an immune response and inflammation, that then contributes to the ocular disease," Kristie says. "So if you block that, then you have much better control of the herpetic disease. And this drug does that much better than pharmaceuticals that are out there now."
• Guinea pigs infected with genital herpes also showed a far lower level of lesion recurrences.
"The interesting thing about each of these model systems is that then, in the animals that are treated, if we look at what the viral genome looks like, we see that it's all packaged up tightly," Kristie says. "It was not able to unpackage, and that's why you have reduced disease, reduced shedding and reduced recurrences."
Researchers are also trying to fight persistent viruses like HIV by trying to activate the virus; then they attack it with drugs or hope the immune system will clear it from the body.
But herpes viruses are reactivated in nerve cells, so they can potentially cause damage to the nervous system when they kick back up, says Harvard Medical School's Dr. David Knipe, chair of the Harvard Program in Virology and a co-author on the new paper.
"So this is the alternative approach, to lock in the latency," he says.
Yet another approach is in the works: a vaccine. Knipe was involved in developing a genital herpes vaccine that Harvard licensed to the pharmaceutical company Sanofi-Pasteur and that is now in initial safety trials at the National Institutes of Health.
It uses a genetically engineered strain of the virus, he says, that can produce an infectious virus only in special cells in the laboratory.
"But when it goes into normal cells in the body, it goes just partway through the replication cycle and then stops," Knipe says. "It makes a lot of viral proteins that can induce immune responses, but not spread to other cells to cause disease."
The vaccine is meant to build immunity in people who have not yet caught the virus, but it may also work to suppress the virus somewhat in people who are already infected, Knipe says.
"We would hope that it would," he says, "but it's not going to cure the infection. It's going to induce a strong enough immune response to combat the virus any time it starts to reactivate."
How soon might the epigenetic approach described in the new paper be tried in humans? Both Kristie and Knipe declined to speculate. But Knipe says he has been thinking that it might first be tried in patients with recurrent herpes viruses that attack the eyes and can damage the cornea, leading to blindness.
Kristie says he hopes the paper will stimulate HIV and other virus researchers to explore similar methods, and he sees potential spillover from cancer drugs that target epigenetics into other fields — like virus research.
"It is a whole new avenue, and it is a very aggressive area of research in terms of development of these compounds that target these different classes of epigenetic enzymes," he says.
