Advertisement
Coronavirus Coverage
Clinical Trials, Speed And Special Technology: Developing The COVID Vaccines
Editor's Note: This is an excerpt from a COVID-19 vaccine special edition of WBUR's weekly coronavirus newsletter. This is part two of three. You can get the other special edition emails, in addition to our weekly vaccine and COVID reporting, in your inbox when you sign up here.
En español, traducido por El Planeta Media.
Last week, we talked about safety, side effects and what not to worry about regarding vaccines and what they do. (Here’s the email if you missed it. And here it is in Spanish, too.) This week, we'll share details about how the vaccines were made, how they work, why the process was different from earlier vaccines, and what that means for the future.
Let's get to the chalkboard.
How vaccines help your immune system
Your immune system has several distinct types of cells, all designed to destroy intruders in your body. It's like the "everybody watches everybody" moment in a casino, where your cells are the blackjack tables, viruses are cheaters, and your immune system is the floor staff, watching for trouble. When the immune system identifies a threat, it moves in like Robert DeNiro and Don Rickles in the "cheater's justice" scene in "Casino" and "gets to work" on the virus.
As we discussed last week, vaccines work by teaching your immune system what to look for and how to attack a specific virus. The first two coronavirus vaccines made available in the U.S. use a relatively new process to do this. They deliver messenger ribonucleic acid, or mRNA, into your body to help teach your immune system how to hobble the virus. And just this past weekend, Johnson & Johnson's vaccine, which uses DNA sequences instead of mRNA to do the same thing, also got the federal green light for emergency use. If you want a less Scorcese-ish explanation of all this, Vox recently released a video explaining how all of these vaccines are made.
The lickety-split nature of COVID-19 vaccine development
Vaccines usually take upwards of a decade to develop, but to fight COVID-19, manufacturers have gone from zero to injections in just about a year. How? Part of it is the use of mRNA, which is much faster to develop in the lab than traditional vaccine technology. But another factor was collapsing the usually multi-step timeline and doing more than one thing at once.
Usually, a company will spend years on research before producing a vaccine candidate. Then that candidate goes through preliminary testing. After that, there are three rounds of human trials:
- The first uses a very small sample in case the candidate vaccine causes some sort of terrible or widespread side effect.
- If it passes muster, the vaccine candidate goes to a second round of trials with more people.
- And finally, a third – with thousands and thousands of people.
After that, it gets into line, RMV-style, waiting for review and authorization by government agencies around the world. Once it’s approved, the company ramps up manufacturing. And finally, those manufactured doses ship out to the waiting public. Rube Goldberg, eat your heart out.
Advertisement
With the coronavirus sweeping the world, there was no time to waste. That's why several of the steps began while others were still underway, including the three trial phases.
If they can do all of this in a year and keep things safe, why aren't all vaccines developed this quickly?
Because developing vaccines costs dump trucks of money. Under normal circumstances, a company can't risk laying out that kind of cash up front without knowing its prototype will work. Things are done one step at a time, and if there are signs the vaccine won't work, the company cuts its losses.
In the face of a pandemic that has devastated human life and crushed economies around the world, the federal government here stepped in with Operation Warp Speed to cover the action; the U.S. provided funding and guaranteed purchases (although Pfizer did not take research and development funding from Warp Speed, they did ink an early deal for vaccine doses that got them needed cash). That greased the skids considerably!
Each vaccine still had to seek what's called "Emergency Use Authorization" (EUA) from the Food & Drug Administration to get special permission to start providing doses before receiving full approval. Here's a helpful explainer from the FDA going into the differences between EUA and full approval.
And Now, A Word From An Actual Science Writer
WBUR’s Angus Chen has closely followed the vaccination process since it started, when the coronavirus genome was first sequenced by Chinese scientists who then shared that data with researchers across the globe. He was good enough to answer some of our questions about the trials:
How was the COVID clinical trial process different from vaccine trials of the past?
The trial process wasn’t all that different except for the fact that it was accelerated. As mentioned earlier, funding from Project Warp Speed allowed companies to put significant capital at risk while research forged ahead.
What's really different is how these vaccines work. There are a lot of different vaccine technologies out there, and some have been around for a long time. For instance, you have attenuated viruses, which are basically "live" viruses that have been altered so they can't replicate or cause illness. Examples are the oral polio vaccine and the chickenpox vaccine. There are also protein, peptide, DNA vaccines and more. There are so many. They all work.
The COVID vaccines from Moderna and Pfizer use modified mRNA for their vaccines, which is a technology that's only been around for about 10 years. This modified mRNA is like a little rogue hacker that sneaks into cells and gets them to start executing a specific code. This code contains the blueprint for a protein. In this case, it is the coronavirus's spike protein. Your body reacts to the spike protein its hacked cells just created and begins making defenses tailored to it. If the body is later invaded by the real coronavirus with a real spike protein, the immune system will be like, "Oh, I've seen this before" and squash it with that nice little arsenal it created with the vaccine.
How did mRNA play a role in Moderna and Pfizer turning around a vaccine so quickly?
Moderna and BioNTech were particularly well-positioned for making mRNA vaccines. The technology itself was pioneered by key scientists in both companies. Katalin Kariko at BioNTech in particular is largely responsible for the technology existing at all. Moderna's name is actually a mash-up of "modified" and "RNA." Since both companies have been focused on mRNA research, they could transition into making a COVID-19 vaccine with this speedy technology faster and better than anybody else.
The mRNA technology itself is also part of the reason why. Since you only need to write new genetic code to make the vaccine, all you need is the genome of the virus. Once BioNTech and Moderna had that sequence, it took about a week for them to actually make the thing. So they could move into Phase 1 clinical trials very fast.
Why were some people, like children and pregnant women, excluded from the trials? What does that mean for vaccine safety among these groups?
Right now, pregnant women are generally excluded from most clinical research. This is a rather controversial topic because there are a ton of pregnant people at any given time, and they still need medicine and vaccines, too.
Regulators and scientists seem to have agreed that for COVID it's better to include those with risk factors like obesity, age, positive for HIV/AIDS etc. than not include them — though not pregnant women. For more information about pregnancy and the coronavirus vaccines, check out this NPR FAQ.
Children weren't included in the original trials for some biological reasons. They have different immune systems from adults, and the coronavirus affects them quite differently. Mainly, they generally don't get as sick.
Companies say they plan to test vaccines in children and are currently looking at how they work for kids ages 12 to 17. The White House Coronavirus Task Force said it’s pushing the inoculation timeline back for grade schoolers until 2022. High schoolers could be vaccinated in the fall.
Were there people with chronic or immunocompromised conditions included?
Yes; the Moderna and Pfizer trials concluded the vaccine is safe for immunocompromised individuals, including those with HIV/AIDS. They did not, however, evaluate efficacy in that population, as they didn't have enough highly immunocompromised individuals. So, it's possible the vaccine doesn't work as well for them.
The thing you might want to watch out for is a history of anaphylaxis and strong allergic reactions to vaccines. If you have that history, then doctors may exclude you from receiving the vaccine because there have been rare cases of people experiencing severe allergic reactions to the first authorized COVID vaccines.
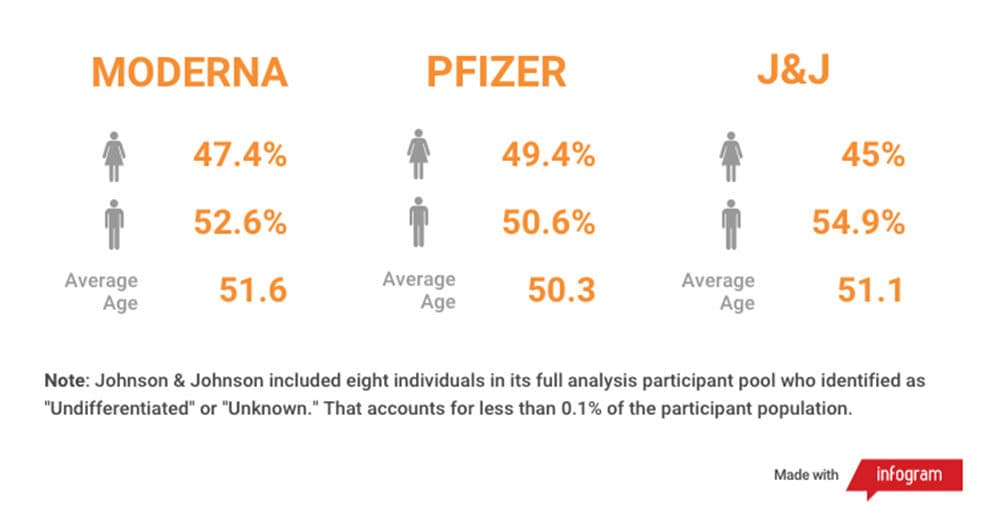
What do we know about the racial, ethnic and gender makeup of who was included in the trials? Were the panels diverse?
Overall, the Moderna and Pfizer trials were close to representative of the U.S. population, but they didn't quite reach proportional representation for several groups, according to the 2019 Census. African Americans, American Indians/Alaska Natives and Asians were not proportionally represented. What’s encouraging is that these trials were very large, and there were still many people from different racial and ethnic backgrounds who participated.
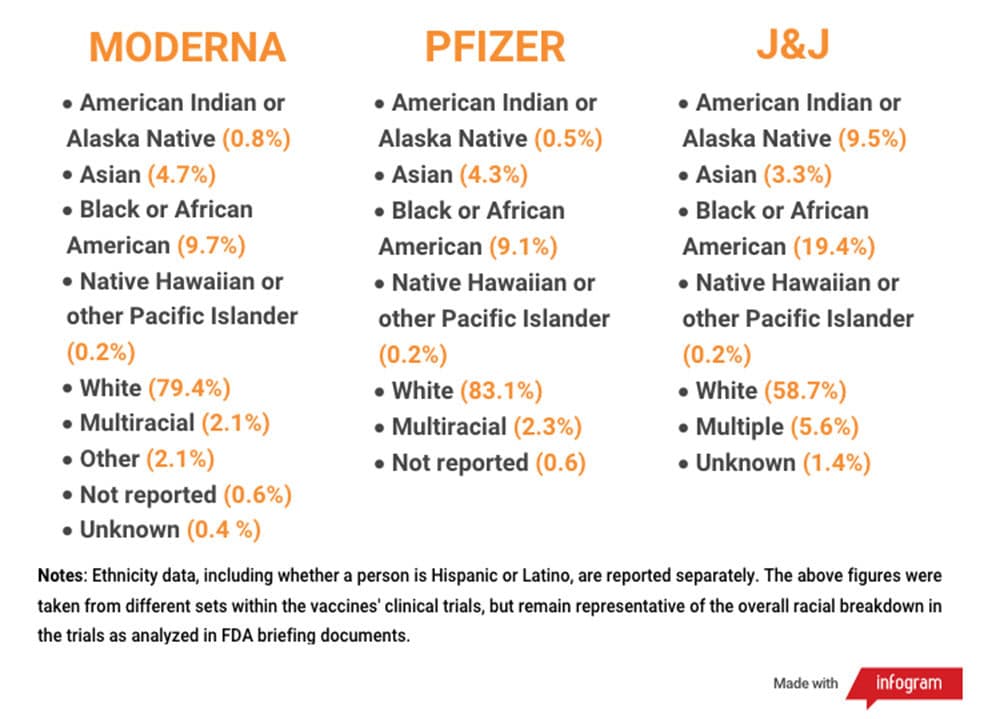
But, if you are a member of a group that has a very small population in the U.S., like Native Hawaiian or other Pacific Islander, then you may not have a very meaningful representation. This group was proportionally represented — but that means only about 60 people took part in the trials. That's not a lot of people for a clinical trial.
It's also worth noting that these vaccines might be taken all over the world, and the clinical trial data doesn't proportionally represent the population of the world. The Johnson & Johnson clinical trial will get closer to that. It was tested in several countries around the world, including Brazil and South Africa, and has a much larger participant population.
Some people raised an eyebrow at the newly approved Johnson & Johnson vaccine, which uses a different technology and has a lower efficacy rate (66%) than the Moderna (94%) and Pfizer (95%) vaccines. Should we be concerned?
The J&J vaccine had some big clinical wins as well. It requires just one dose, is easier to store and distribute, and was over 80% effective at preventing severe disease, including in countries where new, more contagious, coronavirus variants have been identified.
The J&J vaccine uses a different underlying technology. It is a DNA vaccine, and carries genetic instructions for creating the coronavirus spike protein, just like the mRNA vaccines. A notable difference is how the vaccine delivers that genetic code. The mRNA vaccines use a molecular packaging called a lipid nanoparticle. You can think of it as a little biological shrink-wrap that goes around the mRNA. The J&J vaccine uses a modified virus — specifically an adenovirus called ad26. A lot of adenoviruses cause the common cold, but this one has been de-fanged. It cannot replicate or cause illness. It's safe.
Why was there a lower efficacy rate? One possible reason is there are new COVID variants out there that aren't as vulnerable to vaccines built off of this spike protein as the OG coronavirus. The J&J trial started later than those from Moderna and Pfizer and was tested in different settings — including several Latin American countries and South Africa, where highly contagious variants have been identified — so scientists think 66% vs. 95% isn’t the right comparison.
If you look at just the U.S. data, the J&J number then starts getting closer to the Moderna and Pfizer. In the U.S. it was 72% effective. Though the really important thing is that this vaccine was still extremely good at keeping people out of the hospital and the morgue. The more people who are vaccinated, the better.
So really, many experts think this vaccine unfairly gets a bad rap. It's also deliverable in one shot, and it doesn't need mind-numbingly cold temperatures to remain stable. This makes it much easier to distribute.
What do COVID variants mean for vaccine effectiveness?
This is still not totally clear. When Johnson & Johnson tested its vaccine in several countries with higher concentrations of new COVID variants – including South Africa and Brazil – its vaccine was less effective at preventing symptomatic illness. However, it did prevent hospitalization and death at a rate of 100%. Moderna and Pfizer have both reported their vaccines are still effective against known new COVID variants.
The new variants have mutations on the all-important spike protein. In theory, that could change its shape enough that the existing vaccines wouldn’t work as well on these variants. But as Harvard immunologist Tim Springer told me, a few mutations don’t make new variants very distinct from the original Sars-CoV2.
“One or two mutations on the spike change its appearance very little – no more than a mole on a person's face – so the antibodies will still recognize it fine,” he said.
In theory, one day a COVID variant will rear an ugly spiky head that our current vaccines have no effect on. But the good news is that mRNA vaccines can be adapted very easily to any such new variants, and the FDA promises to be nimble, too.
Phew! That was a lot, but we are talking about the rocket-fast response to a global pandemic, so delving into some of the details is important to make sure people feel secure in getting these inoculations. That, we hope, has you ready for our next vaccine special edition in two weeks, when we talk about the Massachusetts vaccine rollout: how it's going and what to expect in the weeks to come.

