Advertisement
Lax Oversight, Lack Of Clinical Trials Mean Some Medical Devices More Likely To Injure Than Cure
Resume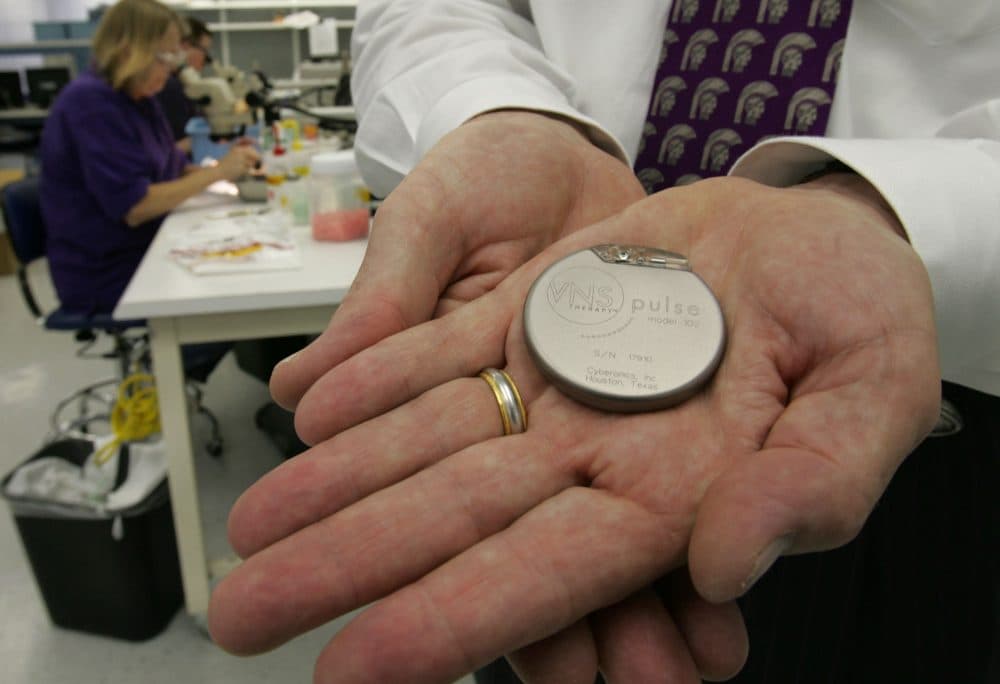
Editor's Note: This segment was rebroadcast on July 24, 2019. That audio is available here.
About 1 in 10 Americans will have some kind of medical device implanted over their lifetime — artificial hips, pace makers, stents, cataract lenses. Drugs must undergo two clinical trials before hitting the market, but fewer than half a percent of high-risk medical devices have undergone that standard. In 2015, the Food and Drug Administration received 16,000 reports of deaths associated with medical devices; one analysis estimated that only 1 percent of device-related deaths are reported to the FDA.
Here & Now's Robin Young discusses with investigative reporter Jeanne Lenzer (@JeanneLenzer1), author of the new book "The Danger Within Us: America's Untested, Unregulated Medical Device Industry and One Man's Battle to Survive It."
- Find more great reads on the Here & Now bookshelf
- Scroll down to read an excerpt from "The Danger Within Us"
Interview Highlights
On "cure as cause"
"One of the problems in medicine is that when something goes wrong, doctors tend to blame the underlying condition or disease that the patient has. And when patients get better, they invariably attribute their improvement to the drug or device that they gave the patient."
On a study that showed from 2008 to 2012, the FDA approved 400 implanted devices of moderate to high risk without any clinical testing
"In fact, the overwhelming majority of medical devices that are on the market, that are implanted in patients, undergo no clinical trials. So of the very high-risk devices capable of causing serious injury or death, those devices, only 5 percent even underwent two clinical trials, and even those clinical trials weren't necessarily trustworthy."
On what's behind the lack of clinical trials for these devices
"What happened was, medical devices didn't come under the purview of FDA until 1976, and at that point there were lots of devices already on the market — things like pacemakers, artificial hips. And so what the FDA did, once devices came under their regulation, was they said, 'OK, all you guys that are already out on the market, you can stay on the market. But we're gonna to divide you up into three classes: class 1 devices are low-risk, class 2 intermediate risk and class 3 are the high-risk implanted devices. So with the class 3 devices, companies were able to keep putting new versions on the market simply by tweaking them and saying, 'Well, there's already a device on the market like this. So we're just gonna add a supplement.' Well, the original device was never put through clinical trials in the first place."
"The overwhelming majority of medical devices that are on the market, that are implanted in patients, undergo no clinical trials."
Jeanne Lenzer
On Dennis Fegan, who had a vagus nerve stimulator implant in his neck to regulate siezures
"Nobody knows why stimulating the vagus nerve should control seizures, and in fact there's really no evidence that it really does. What happened to Dennis was, one night, he was awakened by a pain in his throat, and he thought that that meant a seizure. He knew from prior experience that he'd have this pain in his throat with a seizure. So he got up and he put a vertical mark on his calendar on that date, and when his parents found him the next morning, there were 12 marks on that date. And they came into his house, and he stumbled out of his room and fell face first onto the floor. And then afraid of falling again, he wiggles over, props himself up against a wall with his back to the wall, his legs are splayed in front of them, his jeans are wet with urine, and he promptly passes out again. When the paramedics come, they give him some medicine for epilepsy, and it doesn't work.
"So they rush them to the hospital where the ER doctor looks at his seizures and gives him more, and it still doesn't work. And that's when his doctor notices that Fegan's heart is stopping at exactly three-minute intervals. Fagan wasn't passing out from seizures. He was passing out from lack of oxygen to the brain. The cardiologist takes a look at this and they're all puzzled, and in comes the neurologist who finally explains that Fegan had this VNS device put in him, and the device is set to fire at exactly three-minute intervals."
On whether there's a sense devices will be better tested in the future
"Things are getting worse now. With the 21st Century Cures Act that was passed in 2016, they allowed the level of evidence that's used to approve devices to degrade even further. One of the problems with the device in Dennis Fegan is that that device was known to cause a high rate of deaths, and that's a direct quote from the FDA's own transcripts when they were considering the device, a high rate of deaths among the people who were implanted. They only approved the device conditionally, but they didn't require the manufacturer to tell patients that. Now can you imagine if the company had to say to patients they were intending to implant this device? 'The FDA has approved this only conditionally, meaning they can pull it off the market if we don't prove it's safe, because a lot of people died. Would you still like to have this device implanted in you?' Patients weren't told that.
"What I want to see is honesty in this reporting. It's not that I think all devices are bad. In fact, I'm the beneficiary of an implanted device. What's troubling is we can't tell the difference between good devices and deadly devices. Even people like the doctor you mentioned, who had a hip implant who was an orthopedic surgeon, couldn't tell a good device from a bad device, because there isn't testing. The FDA simply isn't requiring that testing."
Book Excerpt: 'The Danger Within Us'
By Jeanne Lenzer
When I was studying to be a physician associate (PA) at Duke University, I was taught to diagnose and treat common and not-so-common illnesses. As a practicing PA, I wrote prescriptions, performed minor surgical procedures, and made referrals to specialists. I thought that if I stayed on top of new developments in medicine, and was caring and conscientious enough, I could help people. I wouldn’t harm patients. Certainly I wouldn’t kill them.
I was wrong.
For years, I worked in rural emergency rooms, serving as the sole on-site medical provider for patients with everything from sprained ankles to heart attacks and major trauma.
When I saw patients with chest pain, I routinely ordered several drugs, including one called lidocaine, given to prevent or stop extra heartbeats known as premature ventricular contractions (PVCs). Although PVCs can occur occasionally in healthy individuals and aren’t dangerous in themselves, they tend to occur more frequently in heart attack patients, and they have the potential to touch off deadly heart rhythms.
Lidocaine was widely seen as a lifesaving drug. Professional organizations such as the American College of Emergency Physicians and the American Heart Association (AHA) had issued guidelines recommending lidocaine for routine use in patients with acute chest pain.1 And like doctors across the nation, I had faith in the drug, not just because it was recommended but because I’d seen it save the life of a man too young to die.
Mr. R. was in his early forties when he was wheeled into the small rural ER where I was working as the medical provider along with two nurses. One of the nurses recognized him as a local firefighter. He had crushing chest pain and was sweating profusely. I saw the look of terror in his eyes and knew he was wondering, Am I going to die? Within minutes I had ordered oxygen, aspirin, nitroglycerin, and morphine for Mr. R. and was just about to add lidocaine when the alarm on his heart monitor suddenly emitted its high-pitched squeal. I looked up to see the fluorescent green tracing revealing a run of PVCs. I ordered the lidocaine, which the nurse injected into an intravenous line.
The first few minutes after a heart attack are always tense. If one of those PVCs touched off the deadly rhythm known as ventricular fibrillation, Mr. R. was likely to be dead within minutes. The nurse completed the injection. The PVCs persisted. As the alarm continued to screech, I felt beads of sweat collect on my upper lip. Finally the lidocaine took hold. The electrical storm was over. Mr. R.’s heart was beating normally. The alarm fell silent, and I exhaled.
The ability of lidocaine to magically erase these abnormal beats made me secure in the belief that I’d helped a patient cheat death. Over the years, I repeated this process for patients with chest pain many times. It felt good to save lives.
Until I found out I wasn’t saving lives at all.
In the early 1990s, more than a decade after the AHA had recommended lidocaine for chest-pain patients, studies with disturbing results began to emerge. In 1993, one author wrote in the Journal of Emergency Medicine that although the drug could reduce PVCs, “overall mortality may be increased.” A study sponsored by the National Institutes of Health called the Cardiac Arrhythmia Suppression Trial (CAST) found that, although oral drugs related to lidocaine, such as flecainide and propafenone, could suppress PVCs, if they did occur despite the medication, the PVCs were more likely to trigger deadly rhythms. Researchers found that the patients who’d been medicated were actually 3.6 times more likely to die. Thus lidocaine and related drugs could fix the PVCs but kill more patients than they saved in the process. To put it in the vernacular: the operation was a success, but the patient died.
When I learned about the lidocaine study, I was devastated. I was also fascinated. Could it be true that we were killing patients when we were eyewitnesses to so many cures? And if so, if we were really killing patients, why didn’t we see the carnage we’d caused?
I began to search for answers. I stayed up late at night reading about clinical trials and statistics. I attended as many medical conferences as the dollars in my pocket allowed. The more I learned, the more obsessed I became. I wanted to know how and why it was possible for patients and doctors alike to become involved in this massive folie à deux, a dance in which everyone was convinced that the march of medical progress was saving lives even when it wasn’t. How had the best and the brightest doctors, including the guideline writers at the American Heart Association, been fooled? And if we could be fooled by this class of drugs, how many other mirages were we chasing? How many more patients were we harming?
What I learned eventually led me to a very unusual doctor who was able to explain how and why medical illusions like the belief in lidocaine as a lifesaver arise: Dr. Jerome R. Hoffman, then a professor of medicine and emergency medicine at UCLA. Hoffman helped me understand how easy it is for healthcare professionals to make deadly errors despite their best intentions and advanced training, and I’ll share a number of his insights later in this book. These revelations also led me to leave my career in medicine more than a decade ago to become an investigative medical journalist.
Since that time, and especially while writing this book, I’ve been stunned to learn how frequently complications produced by a medical treatment are mistaken for symptoms of the underlying condition—a phenomenon I refer to as cure as cause. The failure to consider a treatment or cure as the cause of a bad outcome means that when a patient dies following a heart attack, we assume that the death was due to the heart attack and not to the medicine we gave. Examples can be found in virtually every specialty, from emergency medicine to psychiatry, cancer care, infectious diseases, and more. Mistaking the ill effects of a treatment as symptoms of the condition being treated is an illusion that is both natural and understandable—and it’s an illusion that the healthcare industry repeatedly exploits to reap enormous profits.
But I was in for yet another shock when I learned the untold story of medical devices and how they, too, can cause the very symptoms they are intended to cure. I began to delve into the rise of the medical device industry and its disturbing role in the healthcare business. Although many concerned citizens have grown distrustful of the pharmaceuticals industry, thanks to the high-profile exposés of dangerous drugs such as Vioxx and Avandia, the same skepticism is largely lacking about implantable devices—from simple devices such as the surgical mesh used for hernias and urinary incontinence to an array of more complex devices such as deep-brain stimulators; spine implants with biologically active bone stimulators; Wi-Fi- enabled pacemakers and defibrillators; breast implants; brain-fluid shunts; intrauterine devices; filters that catch blood clots on their way to the heart; lens implants for cataracts; cadaver bone used for dental surgery; artificial heart valves; gastric bands; artificial hips, knees, and elbows; hormonal implants; cochlear implants; radium seeds; stents to hold coronary, carotid, and renal arteries open; stents that release drugs; and more. The public and doctors often perceive these devices as advanced products of cutting-edge technology that are inert and so, unlike drugs, don’t have serious side effects.
Nothing could be further from the truth.
The reality is that the Food and Drug Administration (FDA) does not require manufacturers to submit even a single clinical trial for the overwhelming majority of high-risk implanted devices it approves. Whereas the standard for approval of a new medicine usually calls for two randomized controlled clinical trials, only 5 percent of high-risk implanted cardiac devices have even partially met that standard. It’s no wonder that many of the millions of patients who have been fitted with implanted medical devices have experienced little or no health benefit as a result—and that others have suffered serious, often fatal, complications from the implants themselves.
It wasn’t long after I became known as a journalist that doctors and patients around the world began to contact me to share their experiences with unsafe drugs and dangerous devices. One story stood out—the story of a Texas man named Dennis Fegan who has spent years battling the after effects of a near-death experience linked to the supposedly safe medical device implanted in him by a well-intentioned doctor. I tell his story in this book not because it is the worst I’ve heard (it isn’t) but rather because it reveals so much about what ails the medical device industry and, more broadly, the health-care system in the US.
As Fegan’s story weaves throughout The Danger Within Us, powerful doctors at the epicenter of modern medicine appear, offering profoundly conflicting views that go to the heart of the way health-care is organized and our perception of what is science and what is illusion in medicine. One of these doctors, Eugene Braunwald, the father of modern cardiology and emeritus professor of medicine at Harvard, has made discoveries that affect millions of people around the world. His career is emblematic of the changing face of healthcare and the emergence of what many call the medical-industrial complex, a network of wealthy and powerful institutions that has dramatically reshaped American healthcare over the past half century. Others, including Dr. Bernard Lown, a Nobel Peace Prize recipient and Harvard cardiologist, and Hoffman, emeritus professor of medicine at UCLA, have called for radical changes in our deeply flawed healthcare system. Their differing perspectives represent alternative future paths that policy makers, healthcare consumers, and concerned citizens urgently need to understand.
Many in the healing professions undoubtedly regret that their service to patients has become so enmeshed with the politics of healthcare and the ideological and partisan clashes it evokes. Yet from a broader perspective, such entanglement is all but inevitable. In the words of Rudolf Virchow, the brilliant nineteenth-century physician credited with bringing scientific rigor to medicine, “Medicine is a social science, and politics [is] nothing more than medicine on a grand scale.”
I hope the stories you’ll read in this book will illustrate Virchow’s insights, which suggest that we cannot achieve a healthy society solely through the application of medical and scientific knowledge and that we will need to address challenging questions about the organization of society, the distribution of resources, and access to information. The answers to these questions have profound implications about who we are as a people and what kind of society we want. Ultimately we have to decide whether healthcare should be treated as a commodity—or a common good.
Excerpted from THE DANGER WITHIN US Copyright © 2017 by Jeanne Lenzer. Used with permission of Little, Brown and Company, New York. All rights reserved.
This article was originally published on January 10, 2018.
This segment aired on January 10, 2018.
